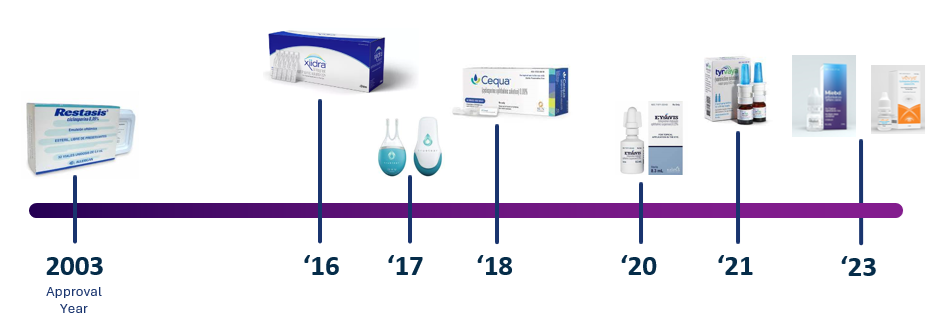
People often comment on the availability of prescription treatments in the USA compared to the UK. While getting approval from the Food and Drug Administration (FDA) is considered more difficult than getting it approved in Europe, the market is bigger and due to the private health system, they can market direct to consumers without the need to convince the National Institute for Health and Care Excellence (NICE) that it is value for money with limited NHS budgets. The pipeline of approved pharmaceuticals has increased over recent years, with 9 products currently approved.
Ophthalmic cyclosporine is an immunomodulator which aims to reduce swelling in the eye to allow for tear production. Restasis was the first approved product, with Cequa and Vevye fast acting formulations.
Lifitegrast (Xiidra) works be a different mechanism to present the release of inflammatory agents. Intranasal tear neurostimulation provides small electric shocks to the inside of the nose to trigger tearing.
Steroids are used extensively to reduce inflammation, but cannot be used long-term in the eyes as the pressure within the eye can rise to dangerous levels; Eysuvis is a low dose steroid option.
Tyrvaya varenicline nasal spray is thought to work by binding to a signalling receptor that activates a neural pathway though the nose to increase tear production.
Meibo perfluorohexyloctane is thought to interact with the surface of the tear film and form a layer to prevent evaporation.
Reference
Patil S, Sawale G, Ghuge S, Sathaye S. Quintessence of currently approved and upcoming treatments for dry eye disease. Graefes Arch Clin Exp Ophthalmol. 2024 Aug 31. doi: 10.1007/s00417-024-06587-7


